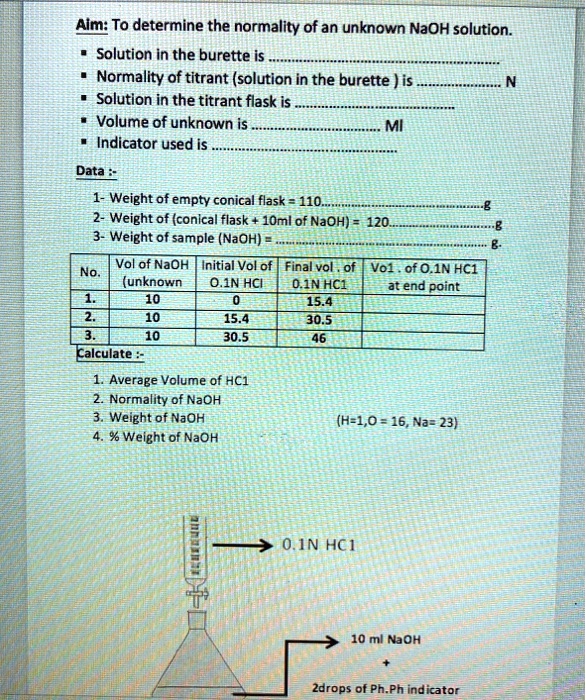
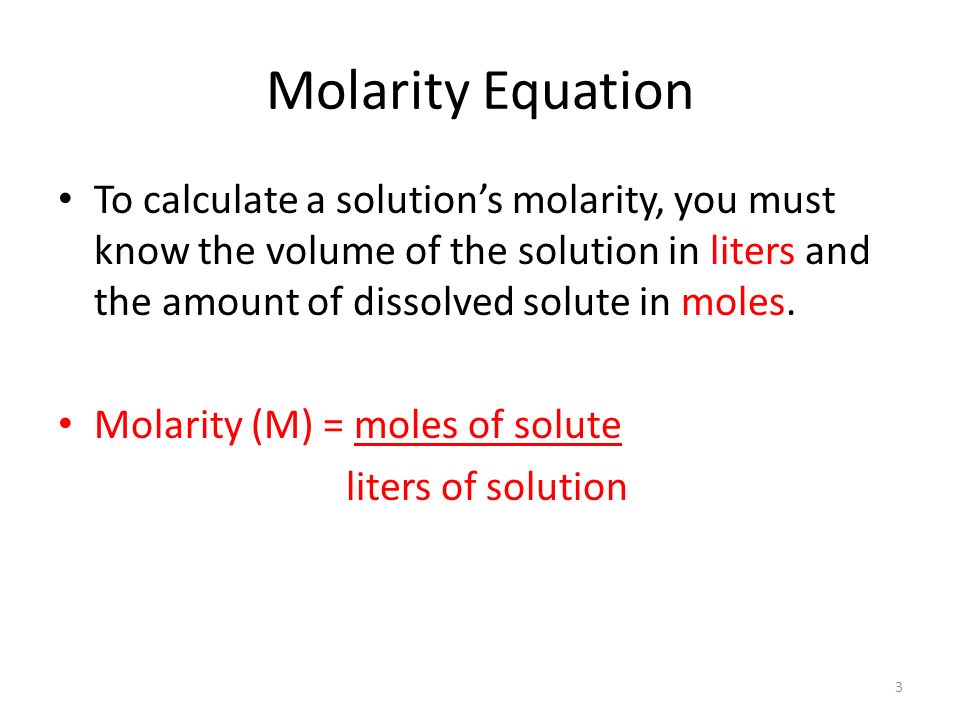
SOLVED: Aim: To determine the normality ofan unknown NaOH solution Solution in the burette is Normality of titrant (solution in the burette ) is Solution in the titrant flask is Volume of

4 g NaOH is added in 100 mL of 0.5 M NaOH solution and solution was made 1 L with addition of water 20 mL of above solution can:

Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to... - YouTube

A sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. What volume of this solution will be required in the preparation of 500ml of a

How to prepare 1% sodium hydroxide (NaOH), 5% NaOH, 10% NaOH solutions: Calculation and Explanation - YouTube

Calculate molality of 2 molar NaOH solution which is 10% by weight - Chemistry - Solutions - 12944633 | Meritnation.com









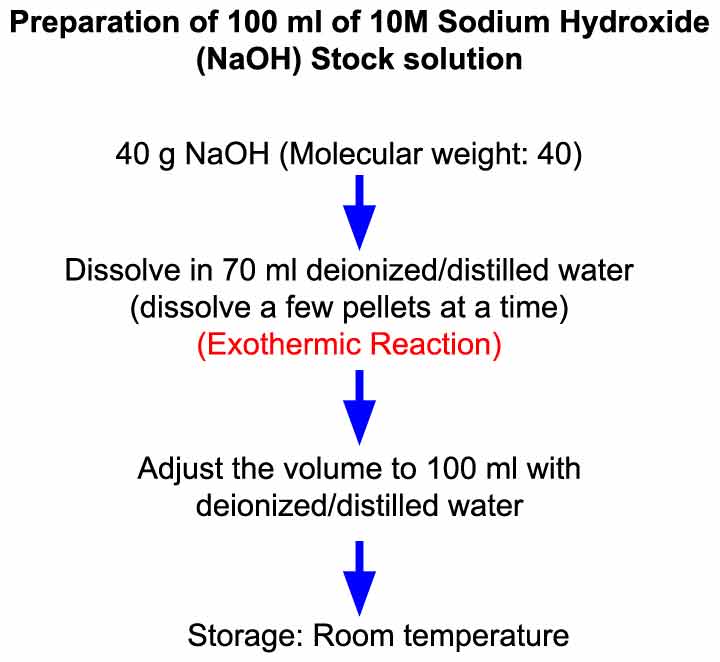
:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)